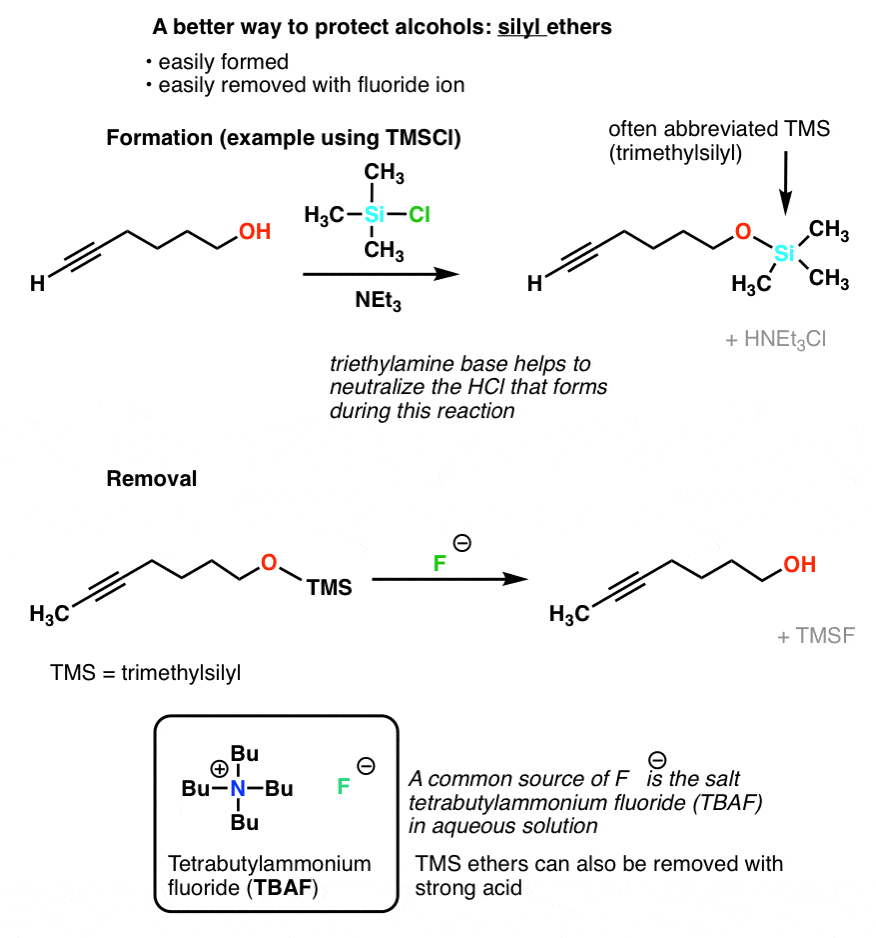
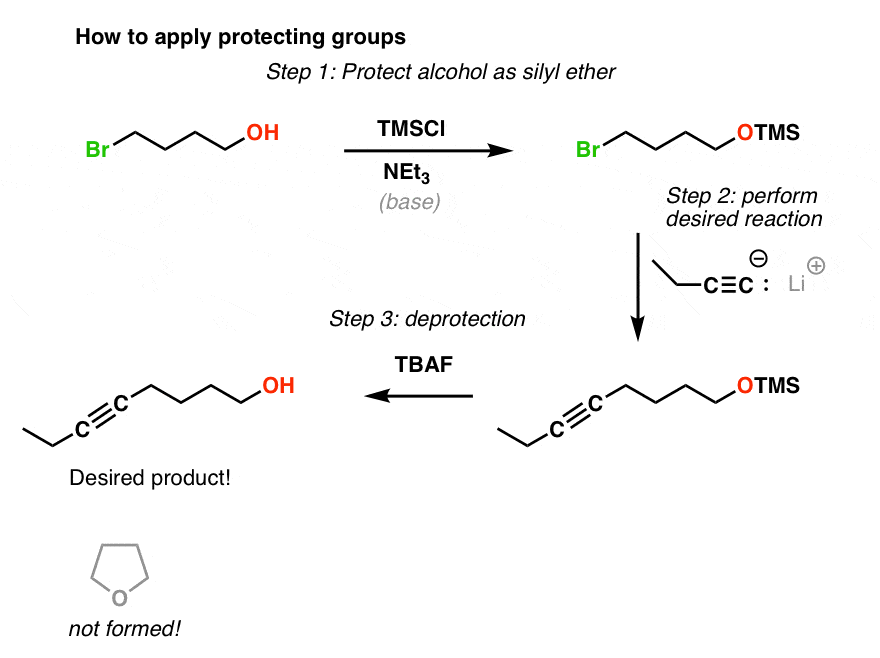
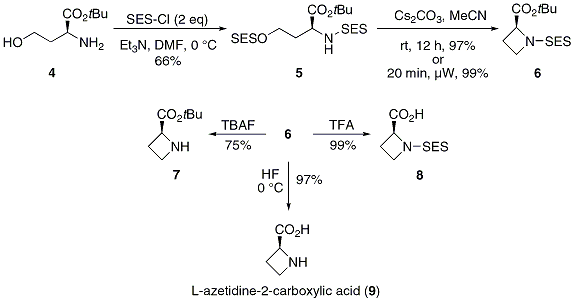
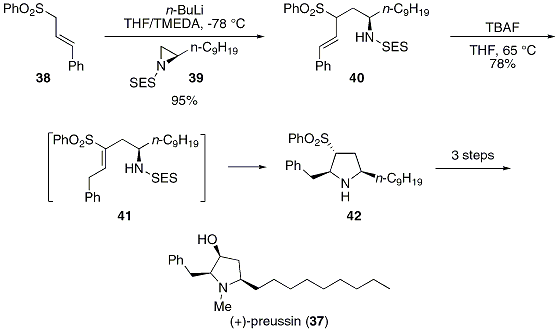
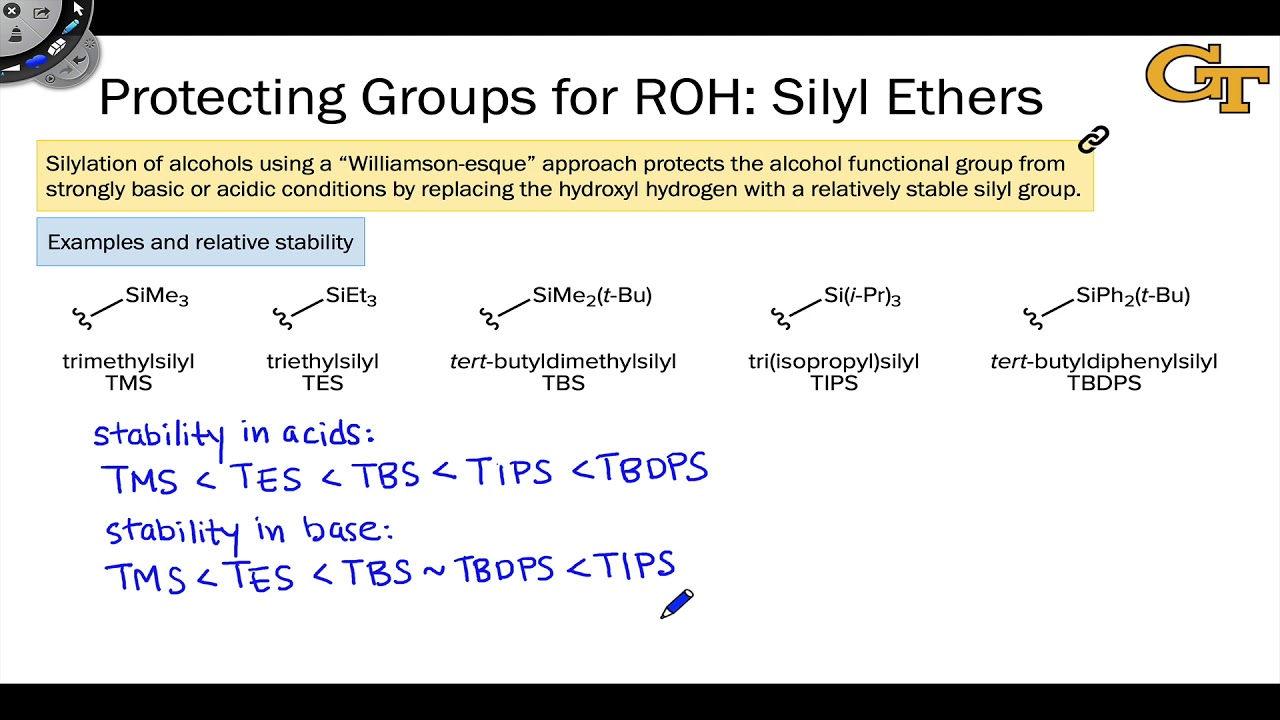
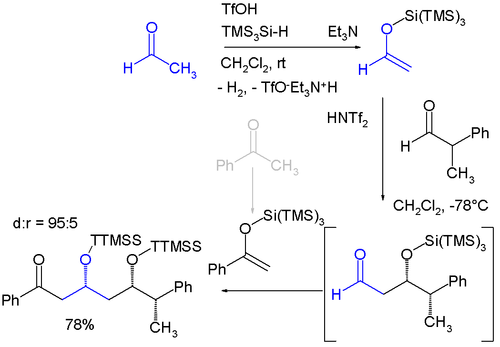
Protecting and Leaving Functions of Trimethylsilyl Groups in Trimethylsilylated Silicates for the Synthesis of Alkoxysiloxane Ol
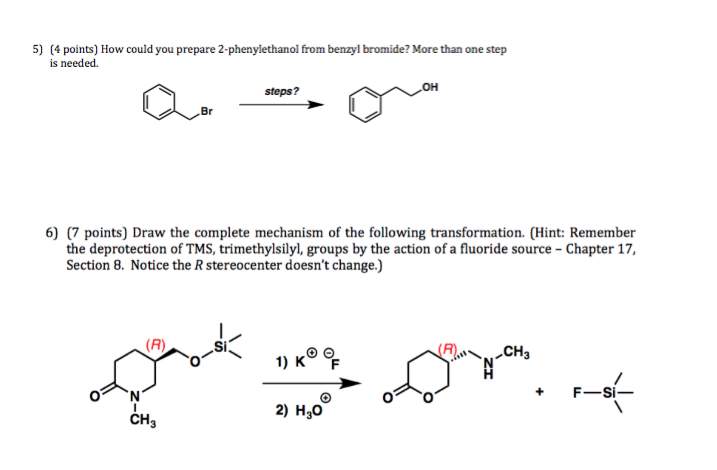
The mechanism of silylether deprotection using a tetra-alkyl ammonium fluoride. | Henry Rzepa's Blog
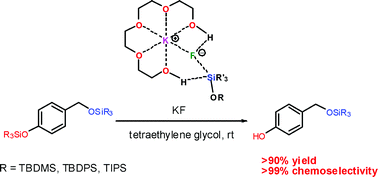
A mild and efficient method for the selective deprotection of silyl ethers using KF in the presence of tetraethylene glycol - Organic & Biomolecular Chemistry (RSC Publishing)

Is there a method to eliminate the trimethylsilyl group from carboxyl or amine ends of a peptide chain after HMDS mediated NCA polymerization?

Is there a method to eliminate the trimethylsilyl group from carboxyl or amine ends of a peptide chain after HMDS mediated NCA polymerization?

A chemoselective deprotection of trimethylsilyl acetylenes catalyzed by silver salts - ScienceDirect