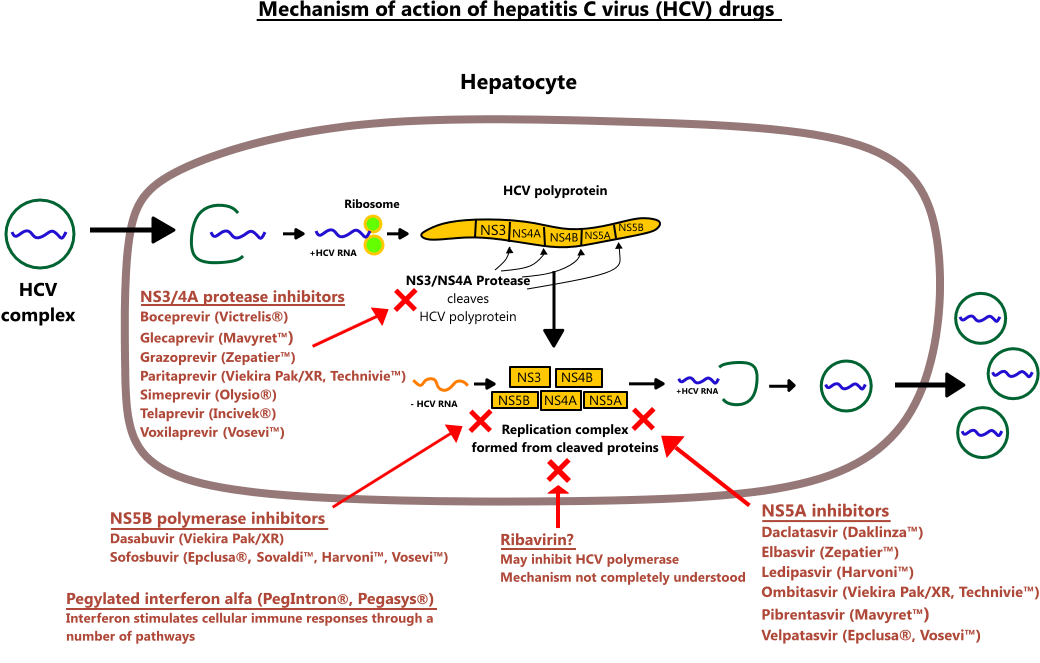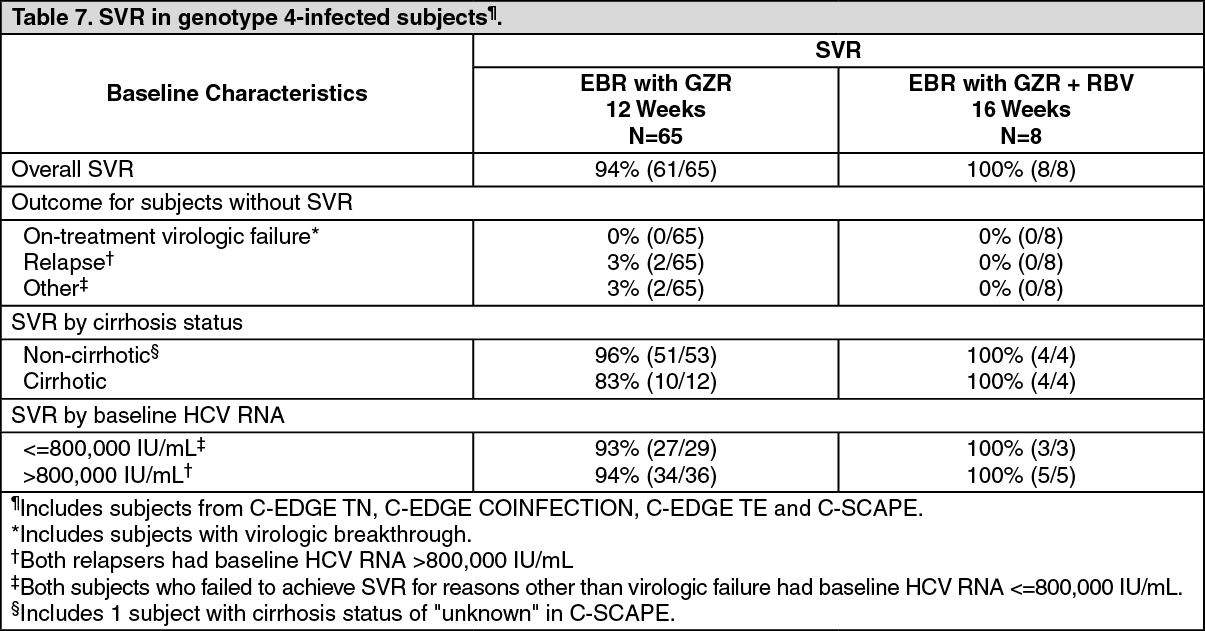
Viruses | Free Full-Text | Mechanisms of Hepatitis C Viral Resistance to Direct Acting Antivirals | HTML

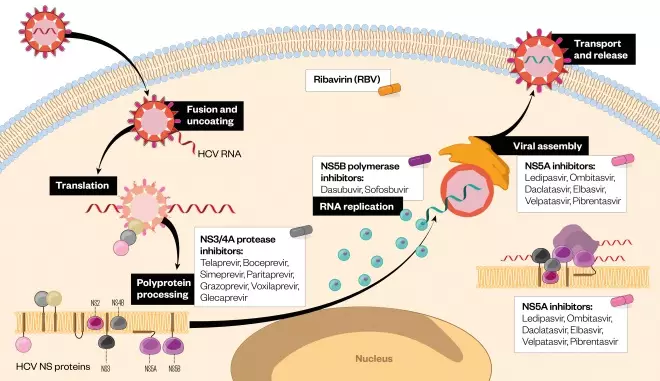
Life cycle of hepatitis C virus (HCV) and targeting points of elbasvir... | Download Scientific Diagram

Zepatier for the Treatment of Chronic Hepatitis C Genotype 1 and 4 Infection - Clinical Trials Arena
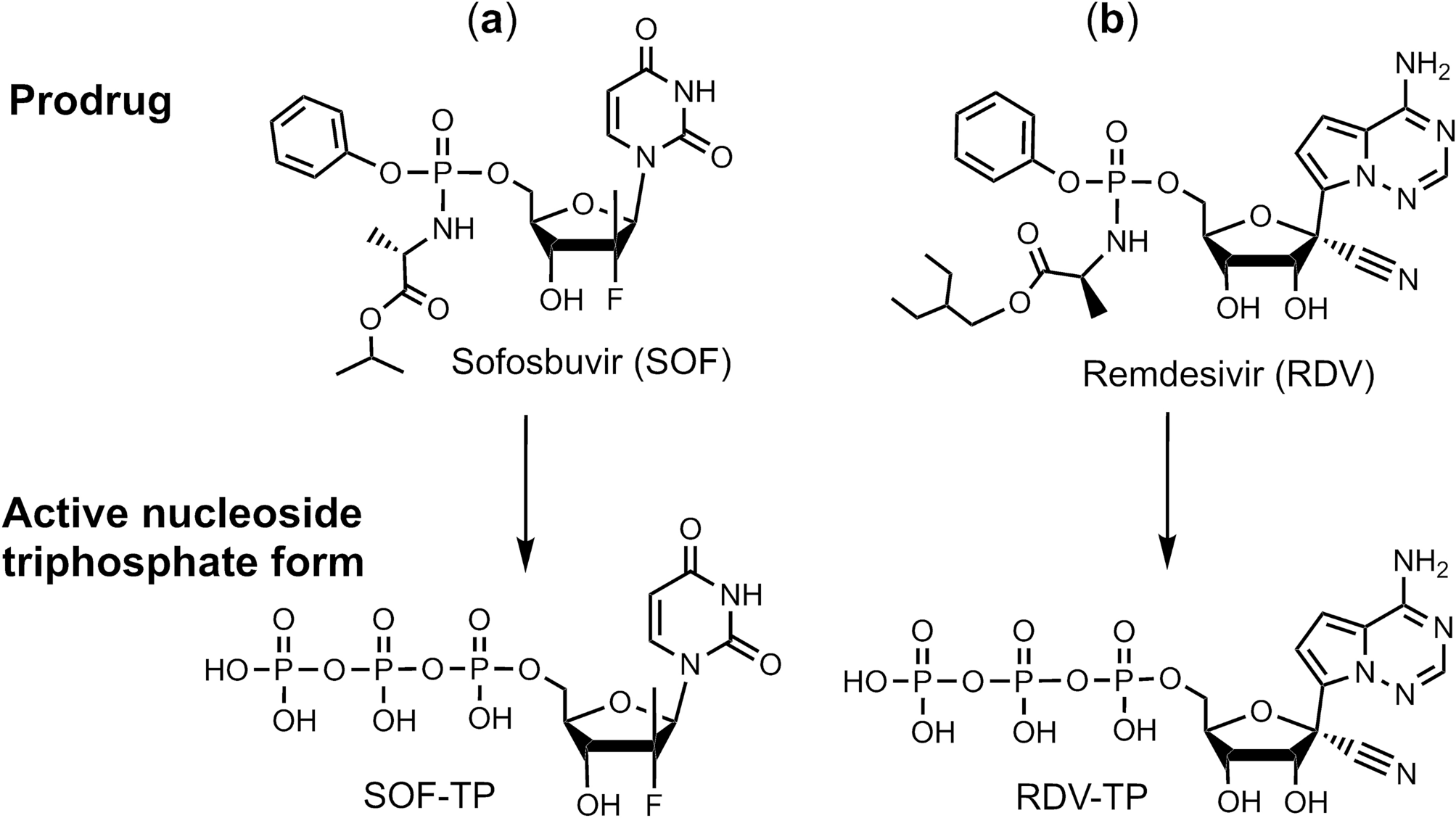
Sofosbuvir terminated RNA is more resistant to SARS-CoV-2 proofreader than RNA terminated by Remdesivir | Scientific Reports
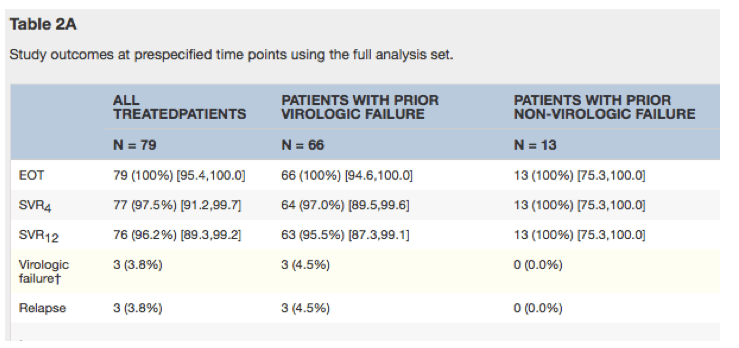
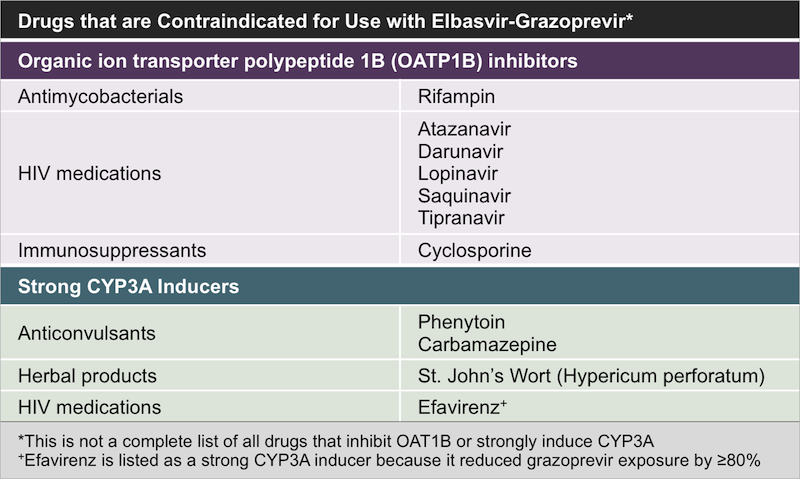
Grazoprevir/Elbasvir plus Ribavirin For Chronic HCV Genotype-1 Infection After Failure of Combination Therapy Containing a Direct-Acting Antiviral Agent
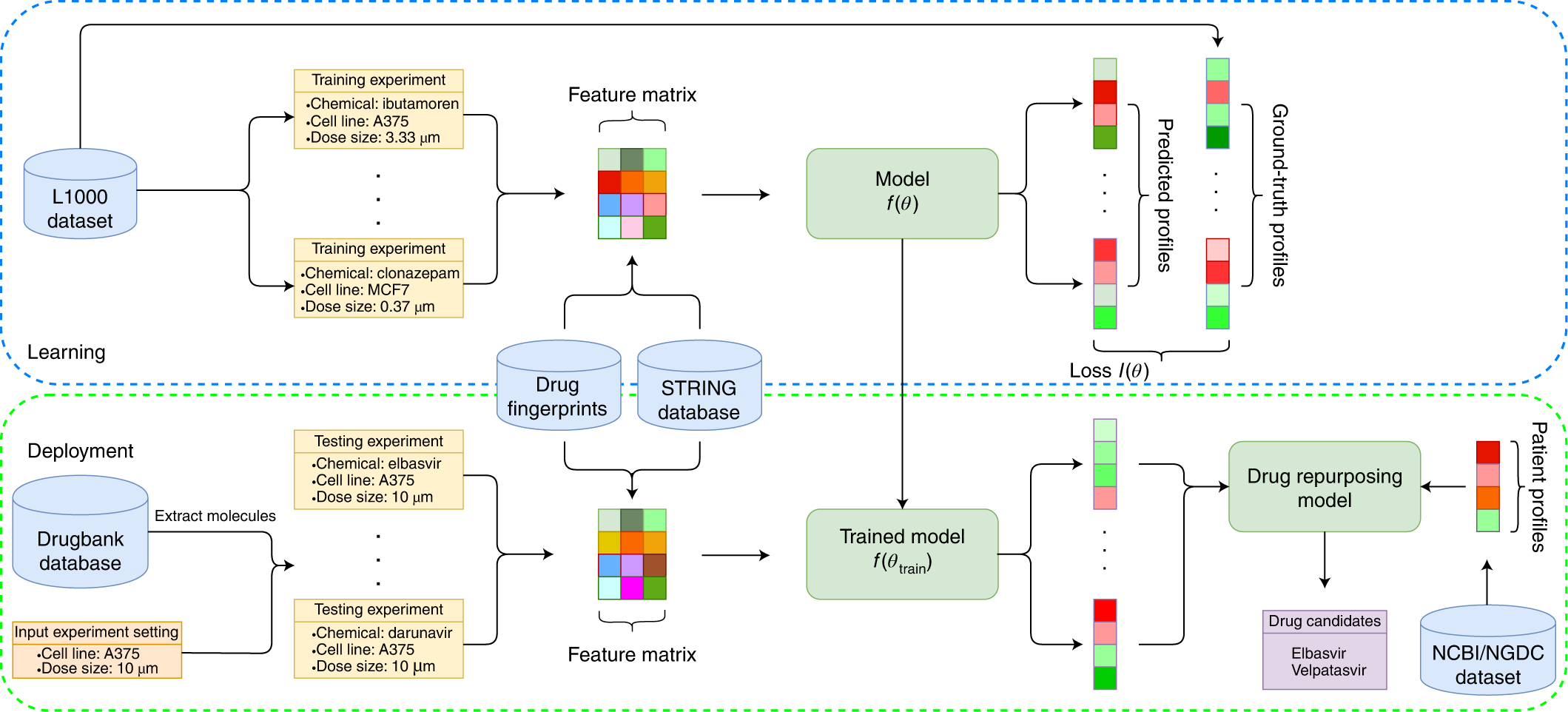
A deep learning framework for high-throughput mechanism-driven phenotype compound screening and its application to COVID-19 drug repurposing | Nature Machine Intelligence
Zepatier for the Treatment of Chronic Hepatitis C Genotype 1 and 4 Infection - Clinical Trials Arena

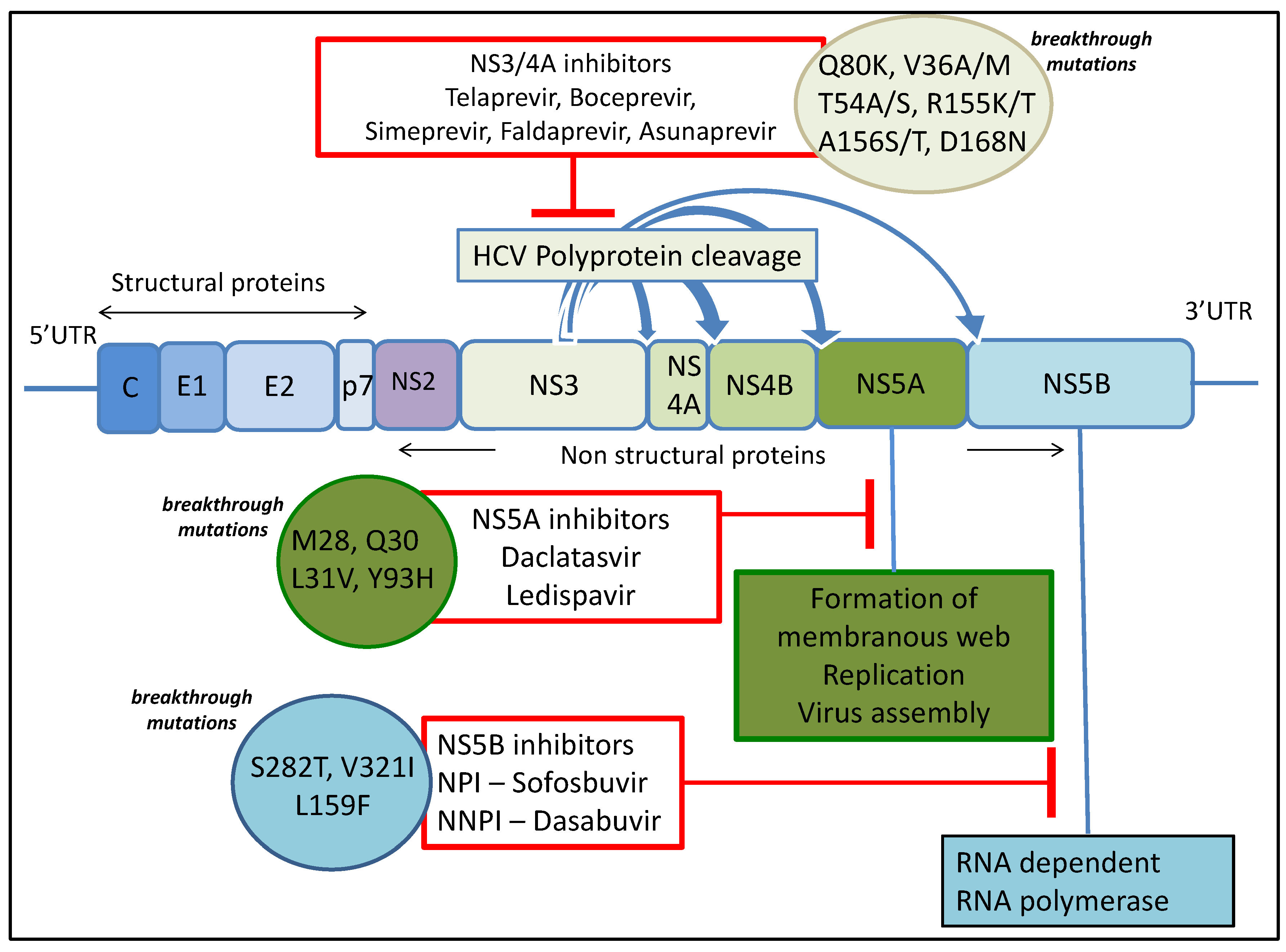
Frontiers | HCV Replicon Systems: Workhorses of Drug Discovery and Resistance | Cellular and Infection Microbiology

Classification of direct-acting antivirals (DAAs) according to their... | Download Scientific Diagram

Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent - Journal of Hepatology